37+ Calculating Limiting Reagent
Given the following reaction which one is the limiting reagent. Limiting reactant is I2.

How To Find Limiting Reactant Quick Easy Examples Practice Problems Practice Questions Youtube
Web In a chemical reaction the reactant that is consumed first and limits how much product can be formed is called the limiting reactant or limiting reagent.

. Web This free limiting reactant calculator assists you to calculate limiting reactant that goes for finishing during the reaction and makes a limited amount of product. Assuming that hot dogs and buns combine in a 1. Web How many complete hot dogs can we make.
240g I2 1 mol I22538gI2 2 mol AlI33 mol I2 000630 mol AlI3. Consider a combustion reaction of say methane. 1 ratio we can make four complete hot dogs.
Lets go through the. Mass-mass calculations can determine how much product is produced and. Web In any chemical reaction you can simply pick one reagent as a candidate for the limiting reagent calculate how many moles of that reagent you have and then.
MolesofAl frac mass g M_r frac 27 27 01 molesofHCl frac mass g M_r frac 9125 365 025. Mass-mass calculations can determine how much product is produced and how much of the. If given mass divide by formula weight to convert to moles this is the mass to mole step.
Web 1 The Steps to Determine the Limiting Reagent or the Limiting Reactant is as Follows. Identify moles of all reactants present. CH 4g 2O2g CO2g 2H 2Og There is lots of oxygen in the atmosphere.
Web Given the chemical equation and the masses of reactants determine the mass of excess reactant and the mass of the limiting reactant required to use up the. Web A quick calculation involving limiting reagents tells you that you can complete the assembly of 40 cars and ship them later that day. You may wish to divide by 1000 to obtain the answer in.
To arrive at the answer most people would. The answer will be in milligrams. You can learn how by.
Web Convert into for both reactants. You are given 100 grams of N 2 and 100 grams of H 2. Web Multiply this result by the MW of the product to determine the expected mass of the product.
Web How To Calculate Limiting Reagents Balance the Equation Before you can find the limiting reagent you must first balance the chemical equation. Given the balanced chemical equation that describes the reaction there. In this video well.
Web The limiting reagent is the reactant that produces the least amount of product. Web Example of a Limiting Reagent Problem. Web The limiting reagent is the reactant that produces the least amount of product.
Web Limiting Reagent Problem Strategies. Web The limiting reagent should be identified to calculate the percentage yield of a reaction. Once we run out of buns well have.
Web 120gAl 1 mol Al2698gAl 2 mol AlI32 mol Al 00445 mol AII3.
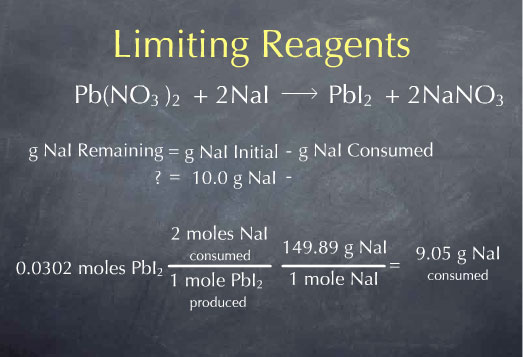
Limiting Reagents
Example Limiting Reagents
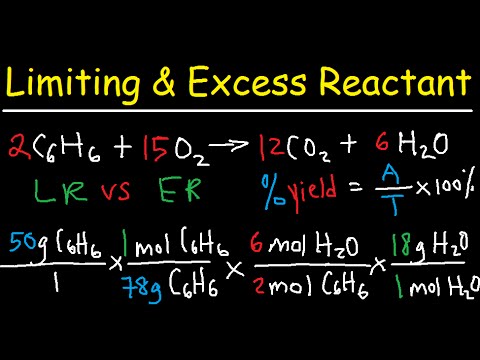
Stoichiometry Limiting Excess Reactant Theoretical Percent Yield Chemistry Youtube

Limiting Reagent Reactant Definition Examples And Problems

Limiting Reactant With 3 Reactants Youtube
Example Limiting Reagents

Stoichiometry Why Do We Only Take Into Account The First Product While Calculating A Limiting Reactant Chemistry Stack Exchange

Design Of Chimeric Histone Deacetylase And Tyrosine Kinase Inhibitors A Series Of Imatinib Hybrides As Potent Inhibitors Of Wild Type And Mutant Bcr Abl Pdgf Rb And Histone Deacetylases Journal Of Medicinal Chemistry

How To Find Limiting Reactants How To Pass Chemistry Youtube

Part 2 Limiting Reactants Percentage Yield Ppt Download

Limiting Reagents Chemistry Libretexts
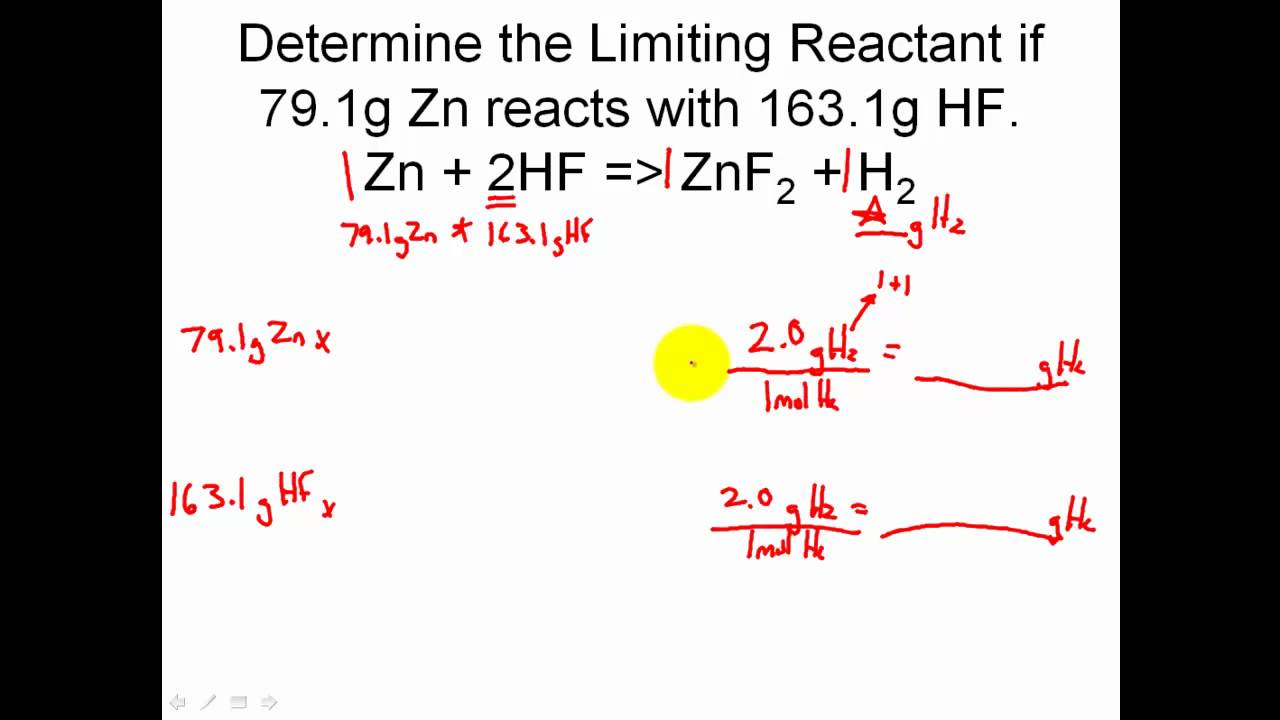
Stoichiometry Solving Limiting Reactant Problems In Stoichiometry Easy Youtube

Chem 101 Dimensional Analysis Limiting Reagent Theoretical Yield Percent Yield Excess Reactant 2 Youtube
:max_bytes(150000):strip_icc()/GettyImages-953952174-e8e65d65fae9438497346d23b11e9cf5.jpg)
Calculating Limiting Reactant Of A Chemical Reaction

Limiting Reactant Practice Problem Advanced Youtube

Stoichiometry Limiting Reagent Ice Box Youtube
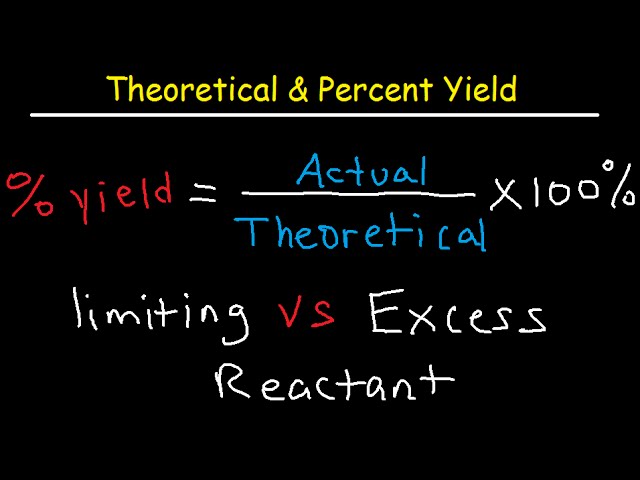
Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube